Stepping Stones to Treating Alzheimer’s Disease: Is Insulin the Key?
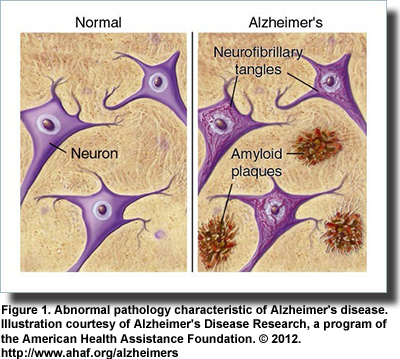 The development and characteristics of Alzheimer’s disease have been extensively described, centered largely on the deposition of sheets of amyloid-beta (Aβ) peptides as extracellular plaques and the protein tau as intracellular neurofibrillary tangles (Figure 1). The disease increases the production of the Aβ peptides in the brain, destroys neurons and synapses, and exhibits several features of metabolic dysregulation, including insulin resistance. It has never been established whether the abnormal pathologies observed in Alzheimer’s disease are a cause or consequence of the condition. Further, definitive diagnosis of Alzheimer’s disease depends on postmortem autopsy. However, the failure of inhibitors of the enzymes responsible for the formation of the abnormal Aβ to improve the welfare of patients raises serious questions about the cause of this disease. Further, the heterogeneous nature of the condition, the involvement of genetics and the diseased brain’s resistance to insulin and insulin-like growth factor (IGF) have expanded thoughts on the origin of Alzheimer’s disease and how it might be treated. A potential breakthrough may be in sight.
The development and characteristics of Alzheimer’s disease have been extensively described, centered largely on the deposition of sheets of amyloid-beta (Aβ) peptides as extracellular plaques and the protein tau as intracellular neurofibrillary tangles (Figure 1). The disease increases the production of the Aβ peptides in the brain, destroys neurons and synapses, and exhibits several features of metabolic dysregulation, including insulin resistance. It has never been established whether the abnormal pathologies observed in Alzheimer’s disease are a cause or consequence of the condition. Further, definitive diagnosis of Alzheimer’s disease depends on postmortem autopsy. However, the failure of inhibitors of the enzymes responsible for the formation of the abnormal Aβ to improve the welfare of patients raises serious questions about the cause of this disease. Further, the heterogeneous nature of the condition, the involvement of genetics and the diseased brain’s resistance to insulin and insulin-like growth factor (IGF) have expanded thoughts on the origin of Alzheimer’s disease and how it might be treated. A potential breakthrough may be in sight.
 Alzheimer’s disease and type 2 diabetes share several metabolic abnormalities, including impaired glucose metabolism, vascular dysfunction, increased oxidative stress, insulin deficiency, insulin resistance and amyloidogenesis. Both the brain and pancreatic islet cells can develop Aβ deposition. Type 2 diabetes is a risk factor for mild cognitive decline, vascular dementia and Alzheimer’s disease and may increase the risk of Alzheimer’s dementia up to 3-fold. It has also been associated with accelerated progression from mildly impaired cognition to dementia. However, it does not cause Alzheimer’s disease. In fact, Alzheimer pathology is observed less frequently in treated diabetics than in non-diabetics. These are among the observations that prompted investigation of insulin in the brain and central nervous system and how insulin resistance develops in individuals with Alzheimer’s disease. Insulin crosses the blood-brain barrier via a transporter mechanism and mediates its effects largely independent of glucose utilization. Its activities in brain are accomplished mainly via two pathways. One is the phosphoinositide-3 kinase (PI3K)/Akt pathway, which is involved in insulin signaling; the other is the Ras/mitogen activated kinase (MAPK) pathway, a chain of proteins that communicate signals from a receptor on the cell surface to receptors on the nucleus. Besides its association with Alzheimer’s disease, insulin resistance in the central nervous system is associated with depressive illness and impaired baroreceptor gain in pregnancy.
Alzheimer’s disease and type 2 diabetes share several metabolic abnormalities, including impaired glucose metabolism, vascular dysfunction, increased oxidative stress, insulin deficiency, insulin resistance and amyloidogenesis. Both the brain and pancreatic islet cells can develop Aβ deposition. Type 2 diabetes is a risk factor for mild cognitive decline, vascular dementia and Alzheimer’s disease and may increase the risk of Alzheimer’s dementia up to 3-fold. It has also been associated with accelerated progression from mildly impaired cognition to dementia. However, it does not cause Alzheimer’s disease. In fact, Alzheimer pathology is observed less frequently in treated diabetics than in non-diabetics. These are among the observations that prompted investigation of insulin in the brain and central nervous system and how insulin resistance develops in individuals with Alzheimer’s disease. Insulin crosses the blood-brain barrier via a transporter mechanism and mediates its effects largely independent of glucose utilization. Its activities in brain are accomplished mainly via two pathways. One is the phosphoinositide-3 kinase (PI3K)/Akt pathway, which is involved in insulin signaling; the other is the Ras/mitogen activated kinase (MAPK) pathway, a chain of proteins that communicate signals from a receptor on the cell surface to receptors on the nucleus. Besides its association with Alzheimer’s disease, insulin resistance in the central nervous system is associated with depressive illness and impaired baroreceptor gain in pregnancy.
 Reduced insulin sensitivity in brain impairs neuroprotection, neuron regeneration and growth factor activity. How peripheral insulin resistance and hyperinsulinemia, characteristics of the metabolic syndrome and type 2 diabetes, relate to cognitive decline and Alzheimer’s disease is unclear. For example, elderly individuals with hyperinsulinemia or insulin resistance, respectively, who were not receiving insulin, had lower scores for cognitive function, delayed memory and attention compared with individuals not having these conditions. Investigators have reported that older individuals with clinical and subclinical diabetes had poorer cognitive performance, executive function and visual memory, and smaller brain volumes compared with individuals without any symptoms of diabetes or insulin resistance. Reduced volume in the hippocampus is considered a marker of neurodegenerative disease. Comparatively smaller gray matter volumes in the medial temporal area and total brain were associated with a 34% lower probability of maintaining cognitive function in elderly adults free of dementia at baseline.
Reduced insulin sensitivity in brain impairs neuroprotection, neuron regeneration and growth factor activity. How peripheral insulin resistance and hyperinsulinemia, characteristics of the metabolic syndrome and type 2 diabetes, relate to cognitive decline and Alzheimer’s disease is unclear. For example, elderly individuals with hyperinsulinemia or insulin resistance, respectively, who were not receiving insulin, had lower scores for cognitive function, delayed memory and attention compared with individuals not having these conditions. Investigators have reported that older individuals with clinical and subclinical diabetes had poorer cognitive performance, executive function and visual memory, and smaller brain volumes compared with individuals without any symptoms of diabetes or insulin resistance. Reduced volume in the hippocampus is considered a marker of neurodegenerative disease. Comparatively smaller gray matter volumes in the medial temporal area and total brain were associated with a 34% lower probability of maintaining cognitive function in elderly adults free of dementia at baseline.
 Impaired insulin signaling appears to be at the core of the abnormalities associated with insulin deficiency in brains with Alzheimer’s disease, just as in type 2 diabetes. In 2005, Eric Steen and colleagues at Brown Medical School, USA, proposed the term “type 3 diabetes” to describe a type of diabetes that selectively affects the brain. They described the extensive abnormalities in insulin and IGF signaling in the brains of individuals with Alzheimer’s disease. These abnormalities were associated with the reduced expression of the genes for these growth factors, their receptors and associated downstream signaling enzymes. On the other hand, there was increased expression of the genes for the amyloid precursor protein and the major tau kinase enzyme, which leads to the formation of the aberrant tau protein. Similar abnormalities were observed in the brains of individuals who died with Alzheimer’s disease, type 2 diabetes, both diseases or none, providing evidence of the overlap in brain abnormalities in both conditions.
Impaired insulin signaling appears to be at the core of the abnormalities associated with insulin deficiency in brains with Alzheimer’s disease, just as in type 2 diabetes. In 2005, Eric Steen and colleagues at Brown Medical School, USA, proposed the term “type 3 diabetes” to describe a type of diabetes that selectively affects the brain. They described the extensive abnormalities in insulin and IGF signaling in the brains of individuals with Alzheimer’s disease. These abnormalities were associated with the reduced expression of the genes for these growth factors, their receptors and associated downstream signaling enzymes. On the other hand, there was increased expression of the genes for the amyloid precursor protein and the major tau kinase enzyme, which leads to the formation of the aberrant tau protein. Similar abnormalities were observed in the brains of individuals who died with Alzheimer’s disease, type 2 diabetes, both diseases or none, providing evidence of the overlap in brain abnormalities in both conditions.

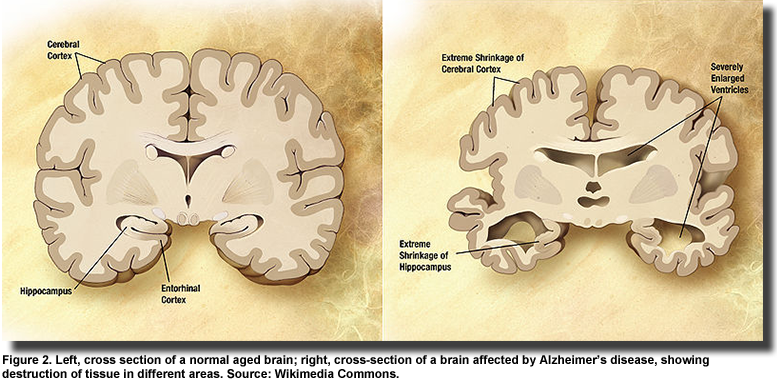 Genes for the insulin and IGF receptors are found in neurons and glial cells throughout the brain. With the progression of Alzheimer’s disease (Figure 2), insulin signaling declines further and insulin and IGF regulated genes are suppressed. Impaired insulin and IGF signaling and regulation are related to the accumulation and toxicity of Aβ, but the discordant observation that diabetic brains accumulate less Aβ, have fewer neuritic plaques, neurofibrillary tangles and lower plaque load in the hippocampus, raise the question of whether impaired insulin and IGF signaling is a cause or consequence of Alzheimer’s disease. Stöhr and colleagues reported that IGF-1 resistance reduces Aβ accumulation and toxicity and promotes survival. Further, these investigators observed that insulin receptor signaling mediates the processing of the amyloid precursor protein and the accumulation of its Aβ peptide products, but does not affect survival. This finding suggests that the insulin receptor is a key regulator of Aβ accumulation. Additional evidence of the protective function of insulin in Alzheimer’s pathology was reported in a study on hippocampal neurons. Aβ oligomers bind to synapses in particular neurons leading to their deterioration and severe memory loss. To accomplish their destructive action, the Aβ oligomers first inhibit critical enzymes in the insulin signaling pathway, which leads to a reduction in insulin receptors and induces oxidative stress. The investigators in this study showed that insulin blocked Aβ binding, prevented the loss of insulin receptors and abolished the development of oxidative stress.
Genes for the insulin and IGF receptors are found in neurons and glial cells throughout the brain. With the progression of Alzheimer’s disease (Figure 2), insulin signaling declines further and insulin and IGF regulated genes are suppressed. Impaired insulin and IGF signaling and regulation are related to the accumulation and toxicity of Aβ, but the discordant observation that diabetic brains accumulate less Aβ, have fewer neuritic plaques, neurofibrillary tangles and lower plaque load in the hippocampus, raise the question of whether impaired insulin and IGF signaling is a cause or consequence of Alzheimer’s disease. Stöhr and colleagues reported that IGF-1 resistance reduces Aβ accumulation and toxicity and promotes survival. Further, these investigators observed that insulin receptor signaling mediates the processing of the amyloid precursor protein and the accumulation of its Aβ peptide products, but does not affect survival. This finding suggests that the insulin receptor is a key regulator of Aβ accumulation. Additional evidence of the protective function of insulin in Alzheimer’s pathology was reported in a study on hippocampal neurons. Aβ oligomers bind to synapses in particular neurons leading to their deterioration and severe memory loss. To accomplish their destructive action, the Aβ oligomers first inhibit critical enzymes in the insulin signaling pathway, which leads to a reduction in insulin receptors and induces oxidative stress. The investigators in this study showed that insulin blocked Aβ binding, prevented the loss of insulin receptors and abolished the development of oxidative stress.
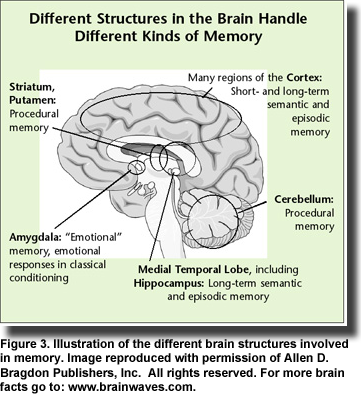 In her comprehensive review of brain insulin resistance and deficiency in Alzheimer’s disease, Suzanne de la Monte presents a strong case for viewing Alzheimer’s disease as a metabolic disease with “progressive derangements in brain glucose utilization and responsiveness to insulin and insulin-like growth factor stimulation.” Evidence points to similarities among obesity, type 2 diabetes, metabolic syndrome and non-alcoholic steatohepatitis, which exhibit brain insulin and IGF resistance and contribute to mild cognitive impairment and Alzheimer’s disease. Mounting evidence suggests that Alzheimer’s disease is associated with insulin resistance and deficiency with or without diabetes or systemic insulin resistance; diabetic patients treated with insulin or hypoglycemic medications have improved memory (Figure 3) and slower progression of Alzheimer’s disease; elderly diabetics treated with insulin have lower densities of Alzheimer’s lesions compared with non-diabetic individuals; insulin treatment is associated with improved memory and cognition in Alzheimer’s patients; and intracerebral or intravenous insulin treatments improve memory, cognition and neurotransmitter function. Thus, the idea of insulin as a therapeutic target in Alzheimer’s disease has gained traction.
In her comprehensive review of brain insulin resistance and deficiency in Alzheimer’s disease, Suzanne de la Monte presents a strong case for viewing Alzheimer’s disease as a metabolic disease with “progressive derangements in brain glucose utilization and responsiveness to insulin and insulin-like growth factor stimulation.” Evidence points to similarities among obesity, type 2 diabetes, metabolic syndrome and non-alcoholic steatohepatitis, which exhibit brain insulin and IGF resistance and contribute to mild cognitive impairment and Alzheimer’s disease. Mounting evidence suggests that Alzheimer’s disease is associated with insulin resistance and deficiency with or without diabetes or systemic insulin resistance; diabetic patients treated with insulin or hypoglycemic medications have improved memory (Figure 3) and slower progression of Alzheimer’s disease; elderly diabetics treated with insulin have lower densities of Alzheimer’s lesions compared with non-diabetic individuals; insulin treatment is associated with improved memory and cognition in Alzheimer’s patients; and intracerebral or intravenous insulin treatments improve memory, cognition and neurotransmitter function. Thus, the idea of insulin as a therapeutic target in Alzheimer’s disease has gained traction.
 As insulin infusion is not a viable long-term therapy and risks hypoglycemia, investigators turned to direct delivery of the hormone via the nose. Intranasal insulin treatment is associated with cognitive benefits and better functional abilities in patients with mildly impaired cognition and in those with mild to moderate Alzheimer’s disease. The treatment was also associated with enhanced learning and memory in healthy adults. At present, there are only a few clinical studies on intranasal insulin treatment and cognitive function, but existing results appear encouraging. Larger, longer trials with individuals across a range of impaired cognition are much needed. Two recent publications now open the doors to more effective treatment of the cognitive and pathological abnormalities in Alzheimer’s disease. Each study builds on the knowledge of defective insulin signaling in this condition. Konrad Talbot and colleagues at the Uni
As insulin infusion is not a viable long-term therapy and risks hypoglycemia, investigators turned to direct delivery of the hormone via the nose. Intranasal insulin treatment is associated with cognitive benefits and better functional abilities in patients with mildly impaired cognition and in those with mild to moderate Alzheimer’s disease. The treatment was also associated with enhanced learning and memory in healthy adults. At present, there are only a few clinical studies on intranasal insulin treatment and cognitive function, but existing results appear encouraging. Larger, longer trials with individuals across a range of impaired cognition are much needed. Two recent publications now open the doors to more effective treatment of the cognitive and pathological abnormalities in Alzheimer’s disease. Each study builds on the knowledge of defective insulin signaling in this condition. Konrad Talbot and colleagues at the Uni
 versity of Pennsylvania, USA, sought to establish that insulin resistance in brain occurs in Alzheimer’s disease independent of type 2 diabetes. They evaluated basal and insulin-stimulated insulin signaling and their associated signaling enzymes upstream and downstream in the insulin receptor-insulin receptor substrate proteins 1 and 2 and the enzymes in the PI3K/Akt pathway in postmortem brain tissue from older adults. Samples from normal and Alzheimer’s patients included tissue from the hippocampal formation, which develops pathology early in the disease, and the cerebellar cortex, which is affected only in late Alzheimer’s disease. The brains of Alzheimer’s patients exhibited insulin and IGF-1 resistance in the absence of diabetes without changes in neuronal glucose uptake. They also demonstrated higher levels of serine-phosphorylated insulin receptor substrate-1 protein, showing that these substances are candidate biomarkers of brain insulin resistance. They were elevated in the hippocampal formation in patients with mildly impaired cognition. The paper reports many more details of their studies, which point to possible treatment of preclinical Alzheimer’s disease. For example, the antidiabetic agents metformin and exenatide, a drug similar to glucagon-like peptide-1, but having a longer half-life in vivo, are both approved for the treatment of type 2 diabetes. The glucagon-like peptide-1 analogues cross the blood-brain barrier, promote neurogenesis and enhance insulin signaling, thus suggesting their usefulness in neurodegenerative diseases.
versity of Pennsylvania, USA, sought to establish that insulin resistance in brain occurs in Alzheimer’s disease independent of type 2 diabetes. They evaluated basal and insulin-stimulated insulin signaling and their associated signaling enzymes upstream and downstream in the insulin receptor-insulin receptor substrate proteins 1 and 2 and the enzymes in the PI3K/Akt pathway in postmortem brain tissue from older adults. Samples from normal and Alzheimer’s patients included tissue from the hippocampal formation, which develops pathology early in the disease, and the cerebellar cortex, which is affected only in late Alzheimer’s disease. The brains of Alzheimer’s patients exhibited insulin and IGF-1 resistance in the absence of diabetes without changes in neuronal glucose uptake. They also demonstrated higher levels of serine-phosphorylated insulin receptor substrate-1 protein, showing that these substances are candidate biomarkers of brain insulin resistance. They were elevated in the hippocampal formation in patients with mildly impaired cognition. The paper reports many more details of their studies, which point to possible treatment of preclinical Alzheimer’s disease. For example, the antidiabetic agents metformin and exenatide, a drug similar to glucagon-like peptide-1, but having a longer half-life in vivo, are both approved for the treatment of type 2 diabetes. The glucagon-like peptide-1 analogues cross the blood-brain barrier, promote neurogenesis and enhance insulin signaling, thus suggesting their usefulness in neurodegenerative diseases.
 The second paper by Theresa Bomfirm and colleagues at the Federal University of Rio De Janeiro, Brazil, reported that insulin signaling is disrupted in Alzheimer’s brains by mechanisms similar to those leading to insulin resistance in diabetes. The investigators showed that Aβ oligomers induce insulin resistance by activating TNF-α and stress kinases, which leads to the serine phosphorylation of the insulin receptor substrate-1 protein. By blocking TNF-α using the drug infliximab they were able to protect neurons against the damage from Aβ oligomers. Further, they showed that exendin-4 (exenatide), which stimulates the insulin signaling pathway through the stimulation of glucagon-like peptide 1 receptors, reversed insulin pathology and improved cognition in a transgenic mouse model of Alzheimer’s disease. These investigators emphasized the importance of inflammation as a mediator of insulin resistance and showed that blocking TNF-α protected neurons against the damaging effects of Aβ oligomers. They suggested that both the strategies, i.e., to blocking TNF-α using infliximab and stimulation of insulin signaling with exendin-4 might reduce Aβ oligomer pathology and prevent memory impairment observed in Alzheimer’s disease.
The second paper by Theresa Bomfirm and colleagues at the Federal University of Rio De Janeiro, Brazil, reported that insulin signaling is disrupted in Alzheimer’s brains by mechanisms similar to those leading to insulin resistance in diabetes. The investigators showed that Aβ oligomers induce insulin resistance by activating TNF-α and stress kinases, which leads to the serine phosphorylation of the insulin receptor substrate-1 protein. By blocking TNF-α using the drug infliximab they were able to protect neurons against the damage from Aβ oligomers. Further, they showed that exendin-4 (exenatide), which stimulates the insulin signaling pathway through the stimulation of glucagon-like peptide 1 receptors, reversed insulin pathology and improved cognition in a transgenic mouse model of Alzheimer’s disease. These investigators emphasized the importance of inflammation as a mediator of insulin resistance and showed that blocking TNF-α protected neurons against the damaging effects of Aβ oligomers. They suggested that both the strategies, i.e., to blocking TNF-α using infliximab and stimulation of insulin signaling with exendin-4 might reduce Aβ oligomer pathology and prevent memory impairment observed in Alzheimer’s disease.
 These studies reveal the diverse abnormalities associated with Alzheimer’s disease and the potential effectiveness of therapeutic strategies targeted to improving insulin signaling, neurogenesis and reducing neuroinflammation. As several antidiabetic drugs are already available, one can speculate that improved treatments for individuals exhibiting cognitive decline and early Alzheimer’s disease might push back the rising tide of dementia. Where do polyunsaturated fatty acids fit in this picture? As described in another article in this issue, higher consumption of n-3 LC-PUFAs was associated with significantly lower concentrations of plasma Aβ42 in healthy non-demented older adults. DHA appears to have multiple effects in affecting the processing of Aβ. Others have described the importance of DHA derivatives, especially neuroprotectin D1, in protecting neurons, promoting cell survival, reducing oxidative stress and modifying the progression of neurodegenerative disease. In a recently reported animal study, diets deficient in n-3 PUFAs and high in fructose were associated with disrupted insulin receptor signaling in the hippocampus, disturbances in brain energy metabolism and reduced synaptic plasticity. The investigators showed that restoring dietary n-3 PUFAs restored normal metabolic function and cognition. Neuroinflammation, a characteristic of acute and chronic neurological disorders, is suppressed by DHA and n-3 LC-PUFAs in animals and cultured cells and in models of brain injury, but studies in humans are lacking. It has been suggested that n-3 LC-PUFAs would be appropriate therapeutic targets in a range of conditions where neuroinflammation occurs. The use of DHA in age-related dementia, particularly Alzheimer’s disease, is supported by several lines of evidence and has been discussed in detail recently. Results in human studies on the effects of n-3 LC-PUFAs and insulin resistance or sensitivity have been mixed. A study in hypertriglyceridemic men suggested that supplementation with 3 grams of DHA per day for 90 days was associated with significant reductions in lipid markers associated with insulin resistance, e.g., small, dense LDL particles, non-esterified fatty acids, but no changes in markers of glucose metabolism. Supplementation with n-3 LC-PUFAs was associated with a reduction in insulin resistance in overweight children, independent of weight loss. In another report, individuals with the metabolic syndrome who consumed a low-fat high-carbohydrate diet supplemented with n-3 LC-PUFAs for 12 weeks experienced a 20.5% reduction in the prevalence of the metabolic syndrome compared with individuals consuming three other types of diet. However, a meta-analysis of 11 randomized trials of n-3 PUFAs and insulin sensitivity concluded that n-3 PUFAs were without effect on insulin sensitivity.
These studies reveal the diverse abnormalities associated with Alzheimer’s disease and the potential effectiveness of therapeutic strategies targeted to improving insulin signaling, neurogenesis and reducing neuroinflammation. As several antidiabetic drugs are already available, one can speculate that improved treatments for individuals exhibiting cognitive decline and early Alzheimer’s disease might push back the rising tide of dementia. Where do polyunsaturated fatty acids fit in this picture? As described in another article in this issue, higher consumption of n-3 LC-PUFAs was associated with significantly lower concentrations of plasma Aβ42 in healthy non-demented older adults. DHA appears to have multiple effects in affecting the processing of Aβ. Others have described the importance of DHA derivatives, especially neuroprotectin D1, in protecting neurons, promoting cell survival, reducing oxidative stress and modifying the progression of neurodegenerative disease. In a recently reported animal study, diets deficient in n-3 PUFAs and high in fructose were associated with disrupted insulin receptor signaling in the hippocampus, disturbances in brain energy metabolism and reduced synaptic plasticity. The investigators showed that restoring dietary n-3 PUFAs restored normal metabolic function and cognition. Neuroinflammation, a characteristic of acute and chronic neurological disorders, is suppressed by DHA and n-3 LC-PUFAs in animals and cultured cells and in models of brain injury, but studies in humans are lacking. It has been suggested that n-3 LC-PUFAs would be appropriate therapeutic targets in a range of conditions where neuroinflammation occurs. The use of DHA in age-related dementia, particularly Alzheimer’s disease, is supported by several lines of evidence and has been discussed in detail recently. Results in human studies on the effects of n-3 LC-PUFAs and insulin resistance or sensitivity have been mixed. A study in hypertriglyceridemic men suggested that supplementation with 3 grams of DHA per day for 90 days was associated with significant reductions in lipid markers associated with insulin resistance, e.g., small, dense LDL particles, non-esterified fatty acids, but no changes in markers of glucose metabolism. Supplementation with n-3 LC-PUFAs was associated with a reduction in insulin resistance in overweight children, independent of weight loss. In another report, individuals with the metabolic syndrome who consumed a low-fat high-carbohydrate diet supplemented with n-3 LC-PUFAs for 12 weeks experienced a 20.5% reduction in the prevalence of the metabolic syndrome compared with individuals consuming three other types of diet. However, a meta-analysis of 11 randomized trials of n-3 PUFAs and insulin sensitivity concluded that n-3 PUFAs were without effect on insulin sensitivity.
 Also described in this issue, there are changes in brain gray matter volumes associated with better cognition. Healthy older adults with the highest intakes of EPA + DHA had greater total brain gray matter volumes and higher cognitive scores compared with individuals consuming the least EPA + DHA. Several studies have reported associations between greater gray matter volumes and better cognitive function. A recent study in animals carrying the gene for human APOE-ε4 gene, which greatly increases the risk of Alzheimer’s disease, reported that animals fed diets enriched in DHA had significantly reduced levels of Aβ in the hippocampus compared with animals fed the non-supplemented diet. The DHA diet also abolished the poorer behavioral performance observed in the non-supplemented animals. Dietary DHA also improves some of the abnormalities observed in the plasma fatty acid profiles in patients with Alzheimer’s disease, but its effect on brain fatty acid profiles may be more complex. A study in patients with mild-to-moderate Alzheimer’s disease reported that supplementation with 2 g of DHA per day for 18 months was associated with significantly increased plasma phospholipid and cerebrospinal fluid DHA concentrations, but had no beneficial effect on cognitive decline, brain atrophy or functional ability. In a recent report, patients with mild-to-moderate Alzheimer’s disease who had impaired blood-brain barrier function were more likely to have dyslipidemia compared with controls. High plasma triglycerides, which can be reduced with n-3 LC-PUFAs, accounted for 22% of the variation in blood-brain barrier integrity. Thus, increased consumption of n-3 LC-PUFAs might improve the integrity of the blood-brain barrier. These studies indicate that DHA supplementation reaches the cerebrospinal fluid and hence the brain and suggests the potential for DHA to improve several abnormalities associated with Alzheimer’s disease and other brain disorders. Many studies have reported associations between low n-3 LC-PUFA intakes or plasma fatty acid status and increased occurrence of impaired cognition and memory loss and risk of Alzheimer’s disease. Various mechanisms from neuroinflammation to altered gene expression to “signalolipidomics” are being studied intensively to explain these associations. Other associations with n-3 LC-PUFAs and improved performance or reduced risk have been reported and are beyond enumeration here. Although amelioration of the clinical symptoms of neurodegenerative disease and cognition may be affected only modestly, the value of adequate intakes of n-3 LC-PUFAs may be greatest in reducing the risk of these conditions in the first place. de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer's disease. Curr Alzheimer Res 2012;9:35-66. [PubMed] Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012;122:1316-1338. [PubMed] Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Aβ oligomers. J Clin Invest 2012;122:1339-1353. [PubMed] Shemesh E, Rudich A, Harman-Boehm I, Cukierman-Yaffe T. Effect of intranasal insulin on cognitive function: a systematic review. J Clin Endocrinol Metab 2012;97:366-376. [PubMed] Bowman GL, Kaye JA, Quinn JF. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr Gerontol Geriatr Res 2012; doi:10.1155/2012/18402. [PubMed]
Also described in this issue, there are changes in brain gray matter volumes associated with better cognition. Healthy older adults with the highest intakes of EPA + DHA had greater total brain gray matter volumes and higher cognitive scores compared with individuals consuming the least EPA + DHA. Several studies have reported associations between greater gray matter volumes and better cognitive function. A recent study in animals carrying the gene for human APOE-ε4 gene, which greatly increases the risk of Alzheimer’s disease, reported that animals fed diets enriched in DHA had significantly reduced levels of Aβ in the hippocampus compared with animals fed the non-supplemented diet. The DHA diet also abolished the poorer behavioral performance observed in the non-supplemented animals. Dietary DHA also improves some of the abnormalities observed in the plasma fatty acid profiles in patients with Alzheimer’s disease, but its effect on brain fatty acid profiles may be more complex. A study in patients with mild-to-moderate Alzheimer’s disease reported that supplementation with 2 g of DHA per day for 18 months was associated with significantly increased plasma phospholipid and cerebrospinal fluid DHA concentrations, but had no beneficial effect on cognitive decline, brain atrophy or functional ability. In a recent report, patients with mild-to-moderate Alzheimer’s disease who had impaired blood-brain barrier function were more likely to have dyslipidemia compared with controls. High plasma triglycerides, which can be reduced with n-3 LC-PUFAs, accounted for 22% of the variation in blood-brain barrier integrity. Thus, increased consumption of n-3 LC-PUFAs might improve the integrity of the blood-brain barrier. These studies indicate that DHA supplementation reaches the cerebrospinal fluid and hence the brain and suggests the potential for DHA to improve several abnormalities associated with Alzheimer’s disease and other brain disorders. Many studies have reported associations between low n-3 LC-PUFA intakes or plasma fatty acid status and increased occurrence of impaired cognition and memory loss and risk of Alzheimer’s disease. Various mechanisms from neuroinflammation to altered gene expression to “signalolipidomics” are being studied intensively to explain these associations. Other associations with n-3 LC-PUFAs and improved performance or reduced risk have been reported and are beyond enumeration here. Although amelioration of the clinical symptoms of neurodegenerative disease and cognition may be affected only modestly, the value of adequate intakes of n-3 LC-PUFAs may be greatest in reducing the risk of these conditions in the first place. de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer's disease. Curr Alzheimer Res 2012;9:35-66. [PubMed] Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012;122:1316-1338. [PubMed] Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Aβ oligomers. J Clin Invest 2012;122:1339-1353. [PubMed] Shemesh E, Rudich A, Harman-Boehm I, Cukierman-Yaffe T. Effect of intranasal insulin on cognitive function: a systematic review. J Clin Endocrinol Metab 2012;97:366-376. [PubMed] Bowman GL, Kaye JA, Quinn JF. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr Gerontol Geriatr Res 2012; doi:10.1155/2012/18402. [PubMed]

