Insight into the Variations in the Individual Response to Supplemental EPA/DHA Intake
The magnitude with which we respond to specific nutrients present in our food is not the same for everyone. Individuals carry significant genetic variability, and also vary in the way genetic information is expressed. Hence our responses to the external world can vary significantly. This variation in response also applies to the absolute needs for long-chain omega-3 fatty acids for health and the effects of their dietary intake on different processes in the body. This is apparent, for example, when we consider that a substantial percentage of people taking omega-3 supplements do not experience a decrease in blood triglyceride (TG) levels, even though a decrease in blood TG levels is one of the hallmarks of omega-3 action if we look at the entire population combined. A mean decrease in plasma TG levels is measurable within a few weeks of increased omega-3 intake, particularly in those with elevated TG levels. It is notable that about a third of individuals do not respond to long-chain omega-3 supplementation with a measurable decrease in TG level.  A proper understanding of the heterogeneity in responses to omega-3 fatty acids has not received much detailed attention thus far. Understanding why some people do respond to omega-3 and others do much less so is of interest. Why increase your omega-3 PUFA intake if you know you will not receive additional benefit at a specific point in your life over your minimally recommended needs for EPA and DHA? On the other hand, a person might switch to taking EPA/DHA if it were clear that he/she would be a high responder and could decide to stop taking a specific prescribed drug instead, to markedly reduce the risk for a specific disorder. In order to be able to make such decisions in the future with a high degree of precision and confidence, a complete understanding of the factors that contribute to this heterogeneity in individual responses to long chain omega-3 is required and the variability should be quantifiable. While we suspect that genetic variability is important, however, we have still to indicate which genes, gene variants, and epigenetic regulation contribute to the individual response to omega-3 supplementation. Now, a study undertaken by Rudkowska and colleagues at Laval University, Quebec, Canada, has examined this question. They undertook the first genome-wide association study (GWAS) to uncover in an unbiased manner those variations in our genome that contribute to the observed variation in the response to EPA/DHA intake. The researchers studied the reduction in blood TG levels as a measurable outcome for the response to supplemental omega-3 intake. Previous studies by this group have addressed the contribution of specific known genetic variations to the TG lowering effect after omega-3 fatty acid intake. Those included specific single-nucleotide polymorphisms (SNPs), one-base pair changes in or between genes that affect gene expression and function, which had been described in prior research. By comparing the presence or absence, or degree of a response or distinguishing characteristic (a measurable phenotype), with the probability of having one or several specific genetic variations, a GWAS offers the possibility to find associations in an unbiased fashion, permitting the discovery of genetic variants that contribute to a specifically studied trait, in this case TG lowering as a result of EPA/DHA supplementation. At this point in time, undertaking a GWAS study potentially permits identifying previously unknown genetic variants, and should identify those variants already known to contribute to a specific response.
A proper understanding of the heterogeneity in responses to omega-3 fatty acids has not received much detailed attention thus far. Understanding why some people do respond to omega-3 and others do much less so is of interest. Why increase your omega-3 PUFA intake if you know you will not receive additional benefit at a specific point in your life over your minimally recommended needs for EPA and DHA? On the other hand, a person might switch to taking EPA/DHA if it were clear that he/she would be a high responder and could decide to stop taking a specific prescribed drug instead, to markedly reduce the risk for a specific disorder. In order to be able to make such decisions in the future with a high degree of precision and confidence, a complete understanding of the factors that contribute to this heterogeneity in individual responses to long chain omega-3 is required and the variability should be quantifiable. While we suspect that genetic variability is important, however, we have still to indicate which genes, gene variants, and epigenetic regulation contribute to the individual response to omega-3 supplementation. Now, a study undertaken by Rudkowska and colleagues at Laval University, Quebec, Canada, has examined this question. They undertook the first genome-wide association study (GWAS) to uncover in an unbiased manner those variations in our genome that contribute to the observed variation in the response to EPA/DHA intake. The researchers studied the reduction in blood TG levels as a measurable outcome for the response to supplemental omega-3 intake. Previous studies by this group have addressed the contribution of specific known genetic variations to the TG lowering effect after omega-3 fatty acid intake. Those included specific single-nucleotide polymorphisms (SNPs), one-base pair changes in or between genes that affect gene expression and function, which had been described in prior research. By comparing the presence or absence, or degree of a response or distinguishing characteristic (a measurable phenotype), with the probability of having one or several specific genetic variations, a GWAS offers the possibility to find associations in an unbiased fashion, permitting the discovery of genetic variants that contribute to a specifically studied trait, in this case TG lowering as a result of EPA/DHA supplementation. At this point in time, undertaking a GWAS study potentially permits identifying previously unknown genetic variants, and should identify those variants already known to contribute to a specific response.  Rudkowska and colleagues identified from a group of study participants, drawn from a Canadian population of overweight/obese men and women, one sub-group of individuals (n=81) that responded to EPA/DHA supplementation with a decrease in plasma TG level of greater or equal than 0.01 mmol/liter, and a second sub-group (n=60) that did not decrease TG. The subjects were asked to take 5 grams a day of fish oil concentrate containing 1.9-2.2 g EPA and 1.1 g DHA, during 6 weeks. The study carefully controlled for the basal intake of food- and supplement-derived omega-3 intake, and established that there were no differences in baseline omega-3 status between the two groups. Blood samples were taken prior to and at the end of the intervention period, and plasma samples prepared for analysis of a number of blood parameters. From genomic DNA extracted from whole blood the frequency of alleles of SNPs was determined using a bead chip technology, permitting the detection of the presence of more than 4.3 million markers corresponding to short DNA sequences covering the human genome. Of these, almost 2.7 million SNPs were tested for statistically significant associations with the responsiveness to EPA/DHA supplementation. The frequency whereby specific DNA sequences were occurring in the responder versus the non-responders was reported as odds ratios, defined as the proportion of individuals in the non-responder group having a specific variation over the proportion of individuals with that variation in the responder group. In order to facilitate identifying those positive associations that might be missed when using a very stringent theoretical likelihood, the researchers employed a statistical threshold that was recently indicated to be more inclusive given that SNPs that are closely located within the genome do not segregate independently. The construction of a Genetic Risk Score (GRS) was established based on the identified genomic variations. Of interest, the study did not stop here, and the value of the identified SNPs in predicting the responsiveness to omega-3 intake was assessed by determining the identified genomic variations in stored DNA samples of a second intervention study, the FINGEN study. This study had been carried out in the UK a few years earlier and had determined that over 30% of study participants did not respond with a decrease in plasma TG level with the daily intake of 1.8 gram EPA+DHA as a supplement over a 8 week period. In a similar fashion responders were defined as people who displayed a reduction in TG level greater than or equal to 0.01 mmol/liter. DNA was genotyped for the identified variants and the GRS was applied to determine the degree of identification of responders versus non-responders based on the measured allele frequencies. The results of the first supplementation study show that when the test population was segregated by responder phenotype, the ingestion of EPA/DHA stimulated a marked reduction in plasma TG level (mean decrease of 0,50 mmol/l) in responders. Responders had a higher average baseline TG level (1.53 mmol/l) than non-responders (1.03 mmol/l), although baseline TG levels in both groups could be considered to be within a range considered normal (below 1.7 mmol/l). Average body weight index between responders and non-responders was the same. The TG level in responders after supplementation reached the baseline TG levels of the non-responders, showing that EPA/DHA lowered TG levels when given to people who have higher levels at the start of the intervention. Of interest, the non-responders actually showed a statistically significant increase in plasma TG level (+0.17 mmol/l), indicating that the nature of responsiveness to EPA/DHA in the responder and non-responder groups was different. In responders, but not in non-responders, the total and high-density lipoprotein (HDL)-bound cholesterol levels increased, and fasting insulin levels decreased. It is important to note that baseline as well as supplement-induced increases in plasma phospholipid levels of EPA, DHA, total omega-3 and total omega-6 PUFA were identical between responders and non-responders. Differences in responsiveness are therefore not due to differences in the absorption or tissue distribution of supplemented omega-3 fatty acids.
Rudkowska and colleagues identified from a group of study participants, drawn from a Canadian population of overweight/obese men and women, one sub-group of individuals (n=81) that responded to EPA/DHA supplementation with a decrease in plasma TG level of greater or equal than 0.01 mmol/liter, and a second sub-group (n=60) that did not decrease TG. The subjects were asked to take 5 grams a day of fish oil concentrate containing 1.9-2.2 g EPA and 1.1 g DHA, during 6 weeks. The study carefully controlled for the basal intake of food- and supplement-derived omega-3 intake, and established that there were no differences in baseline omega-3 status between the two groups. Blood samples were taken prior to and at the end of the intervention period, and plasma samples prepared for analysis of a number of blood parameters. From genomic DNA extracted from whole blood the frequency of alleles of SNPs was determined using a bead chip technology, permitting the detection of the presence of more than 4.3 million markers corresponding to short DNA sequences covering the human genome. Of these, almost 2.7 million SNPs were tested for statistically significant associations with the responsiveness to EPA/DHA supplementation. The frequency whereby specific DNA sequences were occurring in the responder versus the non-responders was reported as odds ratios, defined as the proportion of individuals in the non-responder group having a specific variation over the proportion of individuals with that variation in the responder group. In order to facilitate identifying those positive associations that might be missed when using a very stringent theoretical likelihood, the researchers employed a statistical threshold that was recently indicated to be more inclusive given that SNPs that are closely located within the genome do not segregate independently. The construction of a Genetic Risk Score (GRS) was established based on the identified genomic variations. Of interest, the study did not stop here, and the value of the identified SNPs in predicting the responsiveness to omega-3 intake was assessed by determining the identified genomic variations in stored DNA samples of a second intervention study, the FINGEN study. This study had been carried out in the UK a few years earlier and had determined that over 30% of study participants did not respond with a decrease in plasma TG level with the daily intake of 1.8 gram EPA+DHA as a supplement over a 8 week period. In a similar fashion responders were defined as people who displayed a reduction in TG level greater than or equal to 0.01 mmol/liter. DNA was genotyped for the identified variants and the GRS was applied to determine the degree of identification of responders versus non-responders based on the measured allele frequencies. The results of the first supplementation study show that when the test population was segregated by responder phenotype, the ingestion of EPA/DHA stimulated a marked reduction in plasma TG level (mean decrease of 0,50 mmol/l) in responders. Responders had a higher average baseline TG level (1.53 mmol/l) than non-responders (1.03 mmol/l), although baseline TG levels in both groups could be considered to be within a range considered normal (below 1.7 mmol/l). Average body weight index between responders and non-responders was the same. The TG level in responders after supplementation reached the baseline TG levels of the non-responders, showing that EPA/DHA lowered TG levels when given to people who have higher levels at the start of the intervention. Of interest, the non-responders actually showed a statistically significant increase in plasma TG level (+0.17 mmol/l), indicating that the nature of responsiveness to EPA/DHA in the responder and non-responder groups was different. In responders, but not in non-responders, the total and high-density lipoprotein (HDL)-bound cholesterol levels increased, and fasting insulin levels decreased. It is important to note that baseline as well as supplement-induced increases in plasma phospholipid levels of EPA, DHA, total omega-3 and total omega-6 PUFA were identical between responders and non-responders. Differences in responsiveness are therefore not due to differences in the absorption or tissue distribution of supplemented omega-3 fatty acids. 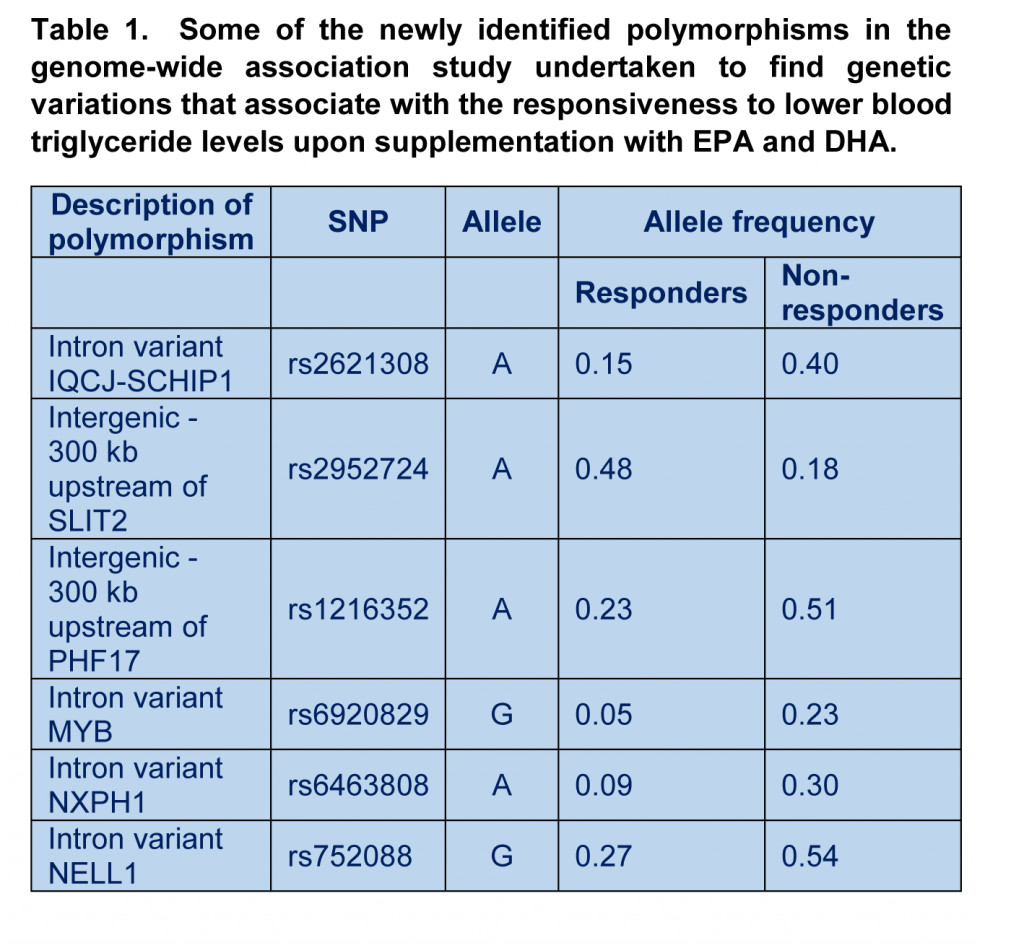 The genomic analysis demonstrated a difference in allele frequency of 13 SNPs (some are shown in Table 1). Several identifed SNPs found in regions of the genome located between genes, potentially modulating the expression of genes located in the vicinity. Several SNPs are reported within genes, such as MYB, NXPH1, NELL1 and IQCJ-SCHIP. These polymorphisms were not previously recognized to play a role in the response to long chain omega-3 fatty acids, but have been reported already to play roles in gene regulation, signaling between adjacent cells, lipoprotein metabolism, and the production of ceramide (a bioactive lipid and important signaling molecule). The results suggest that the responsiveness to EPA/DHA supplementation is intricately linked to other lipid metabolic and signalling pathways. A genetic risk score (GRS) that relates the frequency of the genetic variants to the responder phenotype was developed. The score for each individual falls in a range from -1 to 8 points, attributing points for each allele using 10 of the 13 identified SNPs. A higher score indicates that an individual carries more at risk alleles for a non-responder phenotype, and a lower score, a higher proportion of alleles that increase the probability to respond well to long chain omega-3 supplementation with a decrease in TG levels. The sensitivity of the GRS was 84% (the chance to correctly identify a responder), and the specificity 69% (the chance to correctly identify a non-responder as such). Application of the GRS to the FINGEN replication cohort did not, however, find a very strong predictive power for the newly identified polymorphisms in explaining who was a responder and who was not; while the GRS could identify true responders in 70% of cases, the probability to incorrectly identify a non-responder as such was 75%. Whereas 22% of variation in TG level could be explained by the GRS in the original study, only 2% of the variation could be explained in the validation study, pointing out that additional risk factors still need to be identified that together explain the TG response to omega-3 supplementation in different populations.
The genomic analysis demonstrated a difference in allele frequency of 13 SNPs (some are shown in Table 1). Several identifed SNPs found in regions of the genome located between genes, potentially modulating the expression of genes located in the vicinity. Several SNPs are reported within genes, such as MYB, NXPH1, NELL1 and IQCJ-SCHIP. These polymorphisms were not previously recognized to play a role in the response to long chain omega-3 fatty acids, but have been reported already to play roles in gene regulation, signaling between adjacent cells, lipoprotein metabolism, and the production of ceramide (a bioactive lipid and important signaling molecule). The results suggest that the responsiveness to EPA/DHA supplementation is intricately linked to other lipid metabolic and signalling pathways. A genetic risk score (GRS) that relates the frequency of the genetic variants to the responder phenotype was developed. The score for each individual falls in a range from -1 to 8 points, attributing points for each allele using 10 of the 13 identified SNPs. A higher score indicates that an individual carries more at risk alleles for a non-responder phenotype, and a lower score, a higher proportion of alleles that increase the probability to respond well to long chain omega-3 supplementation with a decrease in TG levels. The sensitivity of the GRS was 84% (the chance to correctly identify a responder), and the specificity 69% (the chance to correctly identify a non-responder as such). Application of the GRS to the FINGEN replication cohort did not, however, find a very strong predictive power for the newly identified polymorphisms in explaining who was a responder and who was not; while the GRS could identify true responders in 70% of cases, the probability to incorrectly identify a non-responder as such was 75%. Whereas 22% of variation in TG level could be explained by the GRS in the original study, only 2% of the variation could be explained in the validation study, pointing out that additional risk factors still need to be identified that together explain the TG response to omega-3 supplementation in different populations.  The GWAS approach by Rudkowska and colleagues shows the power of genome-wide screening approaches to increase our understanding of how the body reacts to specific nutrients, and allows the development of novel predictors of efficacy upon supplementation. Phenotypic variability may also be due to non-genetic factors, for example, long term dietary habits are different between populations and could affect the way genetic information is expressed. It appears that small non-identical conditions in which both studies were carried out with regards to dose, geographic localization, and supplementation period, may affect the predictive value of the GRS in its current form, and reveal that additional risk factors may be identified and incorporated. The distribution of EPA and DHA into specific blood lipids after dietary supplementation also reveals variability in the human population, but it is unusual to observe people that are completely deficient in omega-3 absorption and tissue distribution. Taken together, inter-individual variability in the regulation of some physiological functions by long chain omega-3 PUFA is strongly dependent on genetic influence on how cells respond to omega-3 incorporated in tissues, but non-genetic influences can be expected to contribute as well. This study indicates an attractive way forward to developing a GRS that incorporates identified genetic polymorphisms to predict an individual person´s response to omega-3 intake. In the words of the authors, subjects who decrease their plasma TG levels following omega-3 PUFA supplementation have a different genetic profile compared to individuals who do not respond to the supplementation. The findings in this original study thus set out a path involving follow-up analysis, and possibly new genome-wide studies, to increase the ability and confidence in predicting how we respond individually to dietary and supplemental omega-3 fatty acids. Rudkowska I, Guenard F, Julien P, Couture P, Lemieux S, Barbier O, Calder PC, Minihane AM M D, Vohl MC. Genome-wide association study of the plasma triglyceride response to an n-3 polyunsaturated fatty acid (PUFA) supplementation. J Lipid Res. 2014 May 19. [PubMed] Worth Noting Probe Arrays for Functional Genomics. Bead Arrays. In. NCBI, National Library of Medicine. Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl MC. Polymorphisms in genes involved in fatty acid β-oxidation interact with dietary fat intakes to modulate the plasma TG response to a fish oil supplementation. Nutrients 2014;6(3):1145-1163. [PubMed] Caslake MJ, Miles EA, Kofler BM, Lietz G, Curtis P, Armah CK, Kimber AC, Grew JP, Farrell L, Stannard J, Napper FL, Sala-Vila A, West AL, Mathers JC, Packard C, Williams CM, Calder PC, Minihane AM. Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN Study. Am. J. Clin. Nutr. 2008;88(3):618-629. [PubMed] Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J. Am. Heart Assoc. 2013;2(6):e000513. [PubMed] Nicodemus KK, Liu W, Chase GA, Tsai YY, Fallin MD. Comparison of type I error for multiple test corrections in large single-nucleotide polymorphism studies using principal components versus haplotype blocking algorithms. BMC Genet. 2005;6 (Suppl 1):S78. [PubMed]
The GWAS approach by Rudkowska and colleagues shows the power of genome-wide screening approaches to increase our understanding of how the body reacts to specific nutrients, and allows the development of novel predictors of efficacy upon supplementation. Phenotypic variability may also be due to non-genetic factors, for example, long term dietary habits are different between populations and could affect the way genetic information is expressed. It appears that small non-identical conditions in which both studies were carried out with regards to dose, geographic localization, and supplementation period, may affect the predictive value of the GRS in its current form, and reveal that additional risk factors may be identified and incorporated. The distribution of EPA and DHA into specific blood lipids after dietary supplementation also reveals variability in the human population, but it is unusual to observe people that are completely deficient in omega-3 absorption and tissue distribution. Taken together, inter-individual variability in the regulation of some physiological functions by long chain omega-3 PUFA is strongly dependent on genetic influence on how cells respond to omega-3 incorporated in tissues, but non-genetic influences can be expected to contribute as well. This study indicates an attractive way forward to developing a GRS that incorporates identified genetic polymorphisms to predict an individual person´s response to omega-3 intake. In the words of the authors, subjects who decrease their plasma TG levels following omega-3 PUFA supplementation have a different genetic profile compared to individuals who do not respond to the supplementation. The findings in this original study thus set out a path involving follow-up analysis, and possibly new genome-wide studies, to increase the ability and confidence in predicting how we respond individually to dietary and supplemental omega-3 fatty acids. Rudkowska I, Guenard F, Julien P, Couture P, Lemieux S, Barbier O, Calder PC, Minihane AM M D, Vohl MC. Genome-wide association study of the plasma triglyceride response to an n-3 polyunsaturated fatty acid (PUFA) supplementation. J Lipid Res. 2014 May 19. [PubMed] Worth Noting Probe Arrays for Functional Genomics. Bead Arrays. In. NCBI, National Library of Medicine. Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl MC. Polymorphisms in genes involved in fatty acid β-oxidation interact with dietary fat intakes to modulate the plasma TG response to a fish oil supplementation. Nutrients 2014;6(3):1145-1163. [PubMed] Caslake MJ, Miles EA, Kofler BM, Lietz G, Curtis P, Armah CK, Kimber AC, Grew JP, Farrell L, Stannard J, Napper FL, Sala-Vila A, West AL, Mathers JC, Packard C, Williams CM, Calder PC, Minihane AM. Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN Study. Am. J. Clin. Nutr. 2008;88(3):618-629. [PubMed] Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J. Am. Heart Assoc. 2013;2(6):e000513. [PubMed] Nicodemus KK, Liu W, Chase GA, Tsai YY, Fallin MD. Comparison of type I error for multiple test corrections in large single-nucleotide polymorphism studies using principal components versus haplotype blocking algorithms. BMC Genet. 2005;6 (Suppl 1):S78. [PubMed]
 A proper understanding of the heterogeneity in responses to omega-3 fatty acids has not received much detailed attention thus far. Understanding why some people do respond to omega-3 and others do much less so is of interest. Why increase your omega-3 PUFA intake if you know you will not receive additional benefit at a specific point in your life over your minimally recommended needs for EPA and DHA? On the other hand, a person might switch to taking EPA/DHA if it were clear that he/she would be a high responder and could decide to stop taking a specific prescribed drug instead, to markedly reduce the risk for a specific disorder. In order to be able to make such decisions in the future with a high degree of precision and confidence, a complete understanding of the factors that contribute to this heterogeneity in individual responses to long chain omega-3 is required and the variability should be quantifiable. While we suspect that genetic variability is important, however, we have still to indicate which genes, gene variants, and epigenetic regulation contribute to the individual response to omega-3 supplementation. Now, a study undertaken by Rudkowska and colleagues at Laval University, Quebec, Canada, has examined this question. They undertook the first genome-wide association study (GWAS) to uncover in an unbiased manner those variations in our genome that contribute to the observed variation in the response to EPA/DHA intake. The researchers studied the reduction in blood TG levels as a measurable outcome for the response to supplemental omega-3 intake. Previous studies by this group have addressed the contribution of specific known genetic variations to the TG lowering effect after omega-3 fatty acid intake. Those included specific single-nucleotide polymorphisms (SNPs), one-base pair changes in or between genes that affect gene expression and function, which had been described in prior research. By comparing the presence or absence, or degree of a response or distinguishing characteristic (a measurable phenotype), with the probability of having one or several specific genetic variations, a GWAS offers the possibility to find associations in an unbiased fashion, permitting the discovery of genetic variants that contribute to a specifically studied trait, in this case TG lowering as a result of EPA/DHA supplementation. At this point in time, undertaking a GWAS study potentially permits identifying previously unknown genetic variants, and should identify those variants already known to contribute to a specific response.
A proper understanding of the heterogeneity in responses to omega-3 fatty acids has not received much detailed attention thus far. Understanding why some people do respond to omega-3 and others do much less so is of interest. Why increase your omega-3 PUFA intake if you know you will not receive additional benefit at a specific point in your life over your minimally recommended needs for EPA and DHA? On the other hand, a person might switch to taking EPA/DHA if it were clear that he/she would be a high responder and could decide to stop taking a specific prescribed drug instead, to markedly reduce the risk for a specific disorder. In order to be able to make such decisions in the future with a high degree of precision and confidence, a complete understanding of the factors that contribute to this heterogeneity in individual responses to long chain omega-3 is required and the variability should be quantifiable. While we suspect that genetic variability is important, however, we have still to indicate which genes, gene variants, and epigenetic regulation contribute to the individual response to omega-3 supplementation. Now, a study undertaken by Rudkowska and colleagues at Laval University, Quebec, Canada, has examined this question. They undertook the first genome-wide association study (GWAS) to uncover in an unbiased manner those variations in our genome that contribute to the observed variation in the response to EPA/DHA intake. The researchers studied the reduction in blood TG levels as a measurable outcome for the response to supplemental omega-3 intake. Previous studies by this group have addressed the contribution of specific known genetic variations to the TG lowering effect after omega-3 fatty acid intake. Those included specific single-nucleotide polymorphisms (SNPs), one-base pair changes in or between genes that affect gene expression and function, which had been described in prior research. By comparing the presence or absence, or degree of a response or distinguishing characteristic (a measurable phenotype), with the probability of having one or several specific genetic variations, a GWAS offers the possibility to find associations in an unbiased fashion, permitting the discovery of genetic variants that contribute to a specifically studied trait, in this case TG lowering as a result of EPA/DHA supplementation. At this point in time, undertaking a GWAS study potentially permits identifying previously unknown genetic variants, and should identify those variants already known to contribute to a specific response.  Rudkowska and colleagues identified from a group of study participants, drawn from a Canadian population of overweight/obese men and women, one sub-group of individuals (n=81) that responded to EPA/DHA supplementation with a decrease in plasma TG level of greater or equal than 0.01 mmol/liter, and a second sub-group (n=60) that did not decrease TG. The subjects were asked to take 5 grams a day of fish oil concentrate containing 1.9-2.2 g EPA and 1.1 g DHA, during 6 weeks. The study carefully controlled for the basal intake of food- and supplement-derived omega-3 intake, and established that there were no differences in baseline omega-3 status between the two groups. Blood samples were taken prior to and at the end of the intervention period, and plasma samples prepared for analysis of a number of blood parameters. From genomic DNA extracted from whole blood the frequency of alleles of SNPs was determined using a bead chip technology, permitting the detection of the presence of more than 4.3 million markers corresponding to short DNA sequences covering the human genome. Of these, almost 2.7 million SNPs were tested for statistically significant associations with the responsiveness to EPA/DHA supplementation. The frequency whereby specific DNA sequences were occurring in the responder versus the non-responders was reported as odds ratios, defined as the proportion of individuals in the non-responder group having a specific variation over the proportion of individuals with that variation in the responder group. In order to facilitate identifying those positive associations that might be missed when using a very stringent theoretical likelihood, the researchers employed a statistical threshold that was recently indicated to be more inclusive given that SNPs that are closely located within the genome do not segregate independently. The construction of a Genetic Risk Score (GRS) was established based on the identified genomic variations. Of interest, the study did not stop here, and the value of the identified SNPs in predicting the responsiveness to omega-3 intake was assessed by determining the identified genomic variations in stored DNA samples of a second intervention study, the FINGEN study. This study had been carried out in the UK a few years earlier and had determined that over 30% of study participants did not respond with a decrease in plasma TG level with the daily intake of 1.8 gram EPA+DHA as a supplement over a 8 week period. In a similar fashion responders were defined as people who displayed a reduction in TG level greater than or equal to 0.01 mmol/liter. DNA was genotyped for the identified variants and the GRS was applied to determine the degree of identification of responders versus non-responders based on the measured allele frequencies. The results of the first supplementation study show that when the test population was segregated by responder phenotype, the ingestion of EPA/DHA stimulated a marked reduction in plasma TG level (mean decrease of 0,50 mmol/l) in responders. Responders had a higher average baseline TG level (1.53 mmol/l) than non-responders (1.03 mmol/l), although baseline TG levels in both groups could be considered to be within a range considered normal (below 1.7 mmol/l). Average body weight index between responders and non-responders was the same. The TG level in responders after supplementation reached the baseline TG levels of the non-responders, showing that EPA/DHA lowered TG levels when given to people who have higher levels at the start of the intervention. Of interest, the non-responders actually showed a statistically significant increase in plasma TG level (+0.17 mmol/l), indicating that the nature of responsiveness to EPA/DHA in the responder and non-responder groups was different. In responders, but not in non-responders, the total and high-density lipoprotein (HDL)-bound cholesterol levels increased, and fasting insulin levels decreased. It is important to note that baseline as well as supplement-induced increases in plasma phospholipid levels of EPA, DHA, total omega-3 and total omega-6 PUFA were identical between responders and non-responders. Differences in responsiveness are therefore not due to differences in the absorption or tissue distribution of supplemented omega-3 fatty acids.
Rudkowska and colleagues identified from a group of study participants, drawn from a Canadian population of overweight/obese men and women, one sub-group of individuals (n=81) that responded to EPA/DHA supplementation with a decrease in plasma TG level of greater or equal than 0.01 mmol/liter, and a second sub-group (n=60) that did not decrease TG. The subjects were asked to take 5 grams a day of fish oil concentrate containing 1.9-2.2 g EPA and 1.1 g DHA, during 6 weeks. The study carefully controlled for the basal intake of food- and supplement-derived omega-3 intake, and established that there were no differences in baseline omega-3 status between the two groups. Blood samples were taken prior to and at the end of the intervention period, and plasma samples prepared for analysis of a number of blood parameters. From genomic DNA extracted from whole blood the frequency of alleles of SNPs was determined using a bead chip technology, permitting the detection of the presence of more than 4.3 million markers corresponding to short DNA sequences covering the human genome. Of these, almost 2.7 million SNPs were tested for statistically significant associations with the responsiveness to EPA/DHA supplementation. The frequency whereby specific DNA sequences were occurring in the responder versus the non-responders was reported as odds ratios, defined as the proportion of individuals in the non-responder group having a specific variation over the proportion of individuals with that variation in the responder group. In order to facilitate identifying those positive associations that might be missed when using a very stringent theoretical likelihood, the researchers employed a statistical threshold that was recently indicated to be more inclusive given that SNPs that are closely located within the genome do not segregate independently. The construction of a Genetic Risk Score (GRS) was established based on the identified genomic variations. Of interest, the study did not stop here, and the value of the identified SNPs in predicting the responsiveness to omega-3 intake was assessed by determining the identified genomic variations in stored DNA samples of a second intervention study, the FINGEN study. This study had been carried out in the UK a few years earlier and had determined that over 30% of study participants did not respond with a decrease in plasma TG level with the daily intake of 1.8 gram EPA+DHA as a supplement over a 8 week period. In a similar fashion responders were defined as people who displayed a reduction in TG level greater than or equal to 0.01 mmol/liter. DNA was genotyped for the identified variants and the GRS was applied to determine the degree of identification of responders versus non-responders based on the measured allele frequencies. The results of the first supplementation study show that when the test population was segregated by responder phenotype, the ingestion of EPA/DHA stimulated a marked reduction in plasma TG level (mean decrease of 0,50 mmol/l) in responders. Responders had a higher average baseline TG level (1.53 mmol/l) than non-responders (1.03 mmol/l), although baseline TG levels in both groups could be considered to be within a range considered normal (below 1.7 mmol/l). Average body weight index between responders and non-responders was the same. The TG level in responders after supplementation reached the baseline TG levels of the non-responders, showing that EPA/DHA lowered TG levels when given to people who have higher levels at the start of the intervention. Of interest, the non-responders actually showed a statistically significant increase in plasma TG level (+0.17 mmol/l), indicating that the nature of responsiveness to EPA/DHA in the responder and non-responder groups was different. In responders, but not in non-responders, the total and high-density lipoprotein (HDL)-bound cholesterol levels increased, and fasting insulin levels decreased. It is important to note that baseline as well as supplement-induced increases in plasma phospholipid levels of EPA, DHA, total omega-3 and total omega-6 PUFA were identical between responders and non-responders. Differences in responsiveness are therefore not due to differences in the absorption or tissue distribution of supplemented omega-3 fatty acids.  The genomic analysis demonstrated a difference in allele frequency of 13 SNPs (some are shown in Table 1). Several identifed SNPs found in regions of the genome located between genes, potentially modulating the expression of genes located in the vicinity. Several SNPs are reported within genes, such as MYB, NXPH1, NELL1 and IQCJ-SCHIP. These polymorphisms were not previously recognized to play a role in the response to long chain omega-3 fatty acids, but have been reported already to play roles in gene regulation, signaling between adjacent cells, lipoprotein metabolism, and the production of ceramide (a bioactive lipid and important signaling molecule). The results suggest that the responsiveness to EPA/DHA supplementation is intricately linked to other lipid metabolic and signalling pathways. A genetic risk score (GRS) that relates the frequency of the genetic variants to the responder phenotype was developed. The score for each individual falls in a range from -1 to 8 points, attributing points for each allele using 10 of the 13 identified SNPs. A higher score indicates that an individual carries more at risk alleles for a non-responder phenotype, and a lower score, a higher proportion of alleles that increase the probability to respond well to long chain omega-3 supplementation with a decrease in TG levels. The sensitivity of the GRS was 84% (the chance to correctly identify a responder), and the specificity 69% (the chance to correctly identify a non-responder as such). Application of the GRS to the FINGEN replication cohort did not, however, find a very strong predictive power for the newly identified polymorphisms in explaining who was a responder and who was not; while the GRS could identify true responders in 70% of cases, the probability to incorrectly identify a non-responder as such was 75%. Whereas 22% of variation in TG level could be explained by the GRS in the original study, only 2% of the variation could be explained in the validation study, pointing out that additional risk factors still need to be identified that together explain the TG response to omega-3 supplementation in different populations.
The genomic analysis demonstrated a difference in allele frequency of 13 SNPs (some are shown in Table 1). Several identifed SNPs found in regions of the genome located between genes, potentially modulating the expression of genes located in the vicinity. Several SNPs are reported within genes, such as MYB, NXPH1, NELL1 and IQCJ-SCHIP. These polymorphisms were not previously recognized to play a role in the response to long chain omega-3 fatty acids, but have been reported already to play roles in gene regulation, signaling between adjacent cells, lipoprotein metabolism, and the production of ceramide (a bioactive lipid and important signaling molecule). The results suggest that the responsiveness to EPA/DHA supplementation is intricately linked to other lipid metabolic and signalling pathways. A genetic risk score (GRS) that relates the frequency of the genetic variants to the responder phenotype was developed. The score for each individual falls in a range from -1 to 8 points, attributing points for each allele using 10 of the 13 identified SNPs. A higher score indicates that an individual carries more at risk alleles for a non-responder phenotype, and a lower score, a higher proportion of alleles that increase the probability to respond well to long chain omega-3 supplementation with a decrease in TG levels. The sensitivity of the GRS was 84% (the chance to correctly identify a responder), and the specificity 69% (the chance to correctly identify a non-responder as such). Application of the GRS to the FINGEN replication cohort did not, however, find a very strong predictive power for the newly identified polymorphisms in explaining who was a responder and who was not; while the GRS could identify true responders in 70% of cases, the probability to incorrectly identify a non-responder as such was 75%. Whereas 22% of variation in TG level could be explained by the GRS in the original study, only 2% of the variation could be explained in the validation study, pointing out that additional risk factors still need to be identified that together explain the TG response to omega-3 supplementation in different populations.  The GWAS approach by Rudkowska and colleagues shows the power of genome-wide screening approaches to increase our understanding of how the body reacts to specific nutrients, and allows the development of novel predictors of efficacy upon supplementation. Phenotypic variability may also be due to non-genetic factors, for example, long term dietary habits are different between populations and could affect the way genetic information is expressed. It appears that small non-identical conditions in which both studies were carried out with regards to dose, geographic localization, and supplementation period, may affect the predictive value of the GRS in its current form, and reveal that additional risk factors may be identified and incorporated. The distribution of EPA and DHA into specific blood lipids after dietary supplementation also reveals variability in the human population, but it is unusual to observe people that are completely deficient in omega-3 absorption and tissue distribution. Taken together, inter-individual variability in the regulation of some physiological functions by long chain omega-3 PUFA is strongly dependent on genetic influence on how cells respond to omega-3 incorporated in tissues, but non-genetic influences can be expected to contribute as well. This study indicates an attractive way forward to developing a GRS that incorporates identified genetic polymorphisms to predict an individual person´s response to omega-3 intake. In the words of the authors, subjects who decrease their plasma TG levels following omega-3 PUFA supplementation have a different genetic profile compared to individuals who do not respond to the supplementation. The findings in this original study thus set out a path involving follow-up analysis, and possibly new genome-wide studies, to increase the ability and confidence in predicting how we respond individually to dietary and supplemental omega-3 fatty acids. Rudkowska I, Guenard F, Julien P, Couture P, Lemieux S, Barbier O, Calder PC, Minihane AM M D, Vohl MC. Genome-wide association study of the plasma triglyceride response to an n-3 polyunsaturated fatty acid (PUFA) supplementation. J Lipid Res. 2014 May 19. [PubMed] Worth Noting Probe Arrays for Functional Genomics. Bead Arrays. In. NCBI, National Library of Medicine. Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl MC. Polymorphisms in genes involved in fatty acid β-oxidation interact with dietary fat intakes to modulate the plasma TG response to a fish oil supplementation. Nutrients 2014;6(3):1145-1163. [PubMed] Caslake MJ, Miles EA, Kofler BM, Lietz G, Curtis P, Armah CK, Kimber AC, Grew JP, Farrell L, Stannard J, Napper FL, Sala-Vila A, West AL, Mathers JC, Packard C, Williams CM, Calder PC, Minihane AM. Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN Study. Am. J. Clin. Nutr. 2008;88(3):618-629. [PubMed] Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J. Am. Heart Assoc. 2013;2(6):e000513. [PubMed] Nicodemus KK, Liu W, Chase GA, Tsai YY, Fallin MD. Comparison of type I error for multiple test corrections in large single-nucleotide polymorphism studies using principal components versus haplotype blocking algorithms. BMC Genet. 2005;6 (Suppl 1):S78. [PubMed]
The GWAS approach by Rudkowska and colleagues shows the power of genome-wide screening approaches to increase our understanding of how the body reacts to specific nutrients, and allows the development of novel predictors of efficacy upon supplementation. Phenotypic variability may also be due to non-genetic factors, for example, long term dietary habits are different between populations and could affect the way genetic information is expressed. It appears that small non-identical conditions in which both studies were carried out with regards to dose, geographic localization, and supplementation period, may affect the predictive value of the GRS in its current form, and reveal that additional risk factors may be identified and incorporated. The distribution of EPA and DHA into specific blood lipids after dietary supplementation also reveals variability in the human population, but it is unusual to observe people that are completely deficient in omega-3 absorption and tissue distribution. Taken together, inter-individual variability in the regulation of some physiological functions by long chain omega-3 PUFA is strongly dependent on genetic influence on how cells respond to omega-3 incorporated in tissues, but non-genetic influences can be expected to contribute as well. This study indicates an attractive way forward to developing a GRS that incorporates identified genetic polymorphisms to predict an individual person´s response to omega-3 intake. In the words of the authors, subjects who decrease their plasma TG levels following omega-3 PUFA supplementation have a different genetic profile compared to individuals who do not respond to the supplementation. The findings in this original study thus set out a path involving follow-up analysis, and possibly new genome-wide studies, to increase the ability and confidence in predicting how we respond individually to dietary and supplemental omega-3 fatty acids. Rudkowska I, Guenard F, Julien P, Couture P, Lemieux S, Barbier O, Calder PC, Minihane AM M D, Vohl MC. Genome-wide association study of the plasma triglyceride response to an n-3 polyunsaturated fatty acid (PUFA) supplementation. J Lipid Res. 2014 May 19. [PubMed] Worth Noting Probe Arrays for Functional Genomics. Bead Arrays. In. NCBI, National Library of Medicine. Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl MC. Polymorphisms in genes involved in fatty acid β-oxidation interact with dietary fat intakes to modulate the plasma TG response to a fish oil supplementation. Nutrients 2014;6(3):1145-1163. [PubMed] Caslake MJ, Miles EA, Kofler BM, Lietz G, Curtis P, Armah CK, Kimber AC, Grew JP, Farrell L, Stannard J, Napper FL, Sala-Vila A, West AL, Mathers JC, Packard C, Williams CM, Calder PC, Minihane AM. Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN Study. Am. J. Clin. Nutr. 2008;88(3):618-629. [PubMed] Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J. Am. Heart Assoc. 2013;2(6):e000513. [PubMed] Nicodemus KK, Liu W, Chase GA, Tsai YY, Fallin MD. Comparison of type I error for multiple test corrections in large single-nucleotide polymorphism studies using principal components versus haplotype blocking algorithms. BMC Genet. 2005;6 (Suppl 1):S78. [PubMed]

